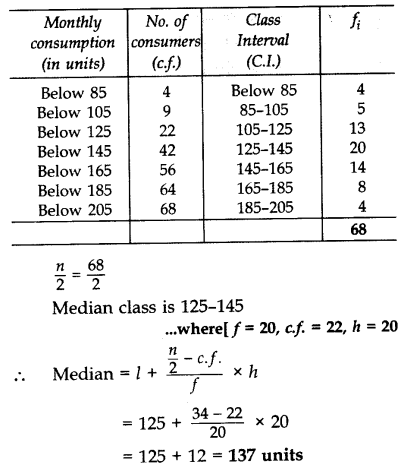
As formulas are entirely constituted with symbols of various types many symbols are needed for expressing all mathematics. It informs which elements are present in a compound and their relative percentages.
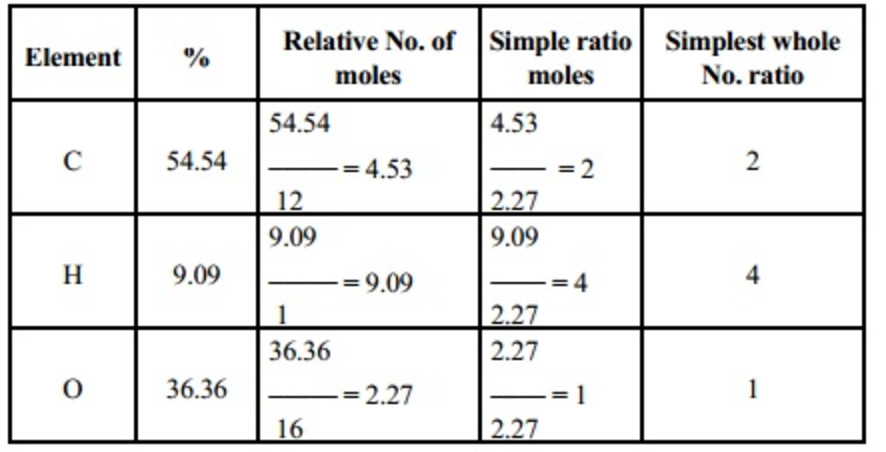
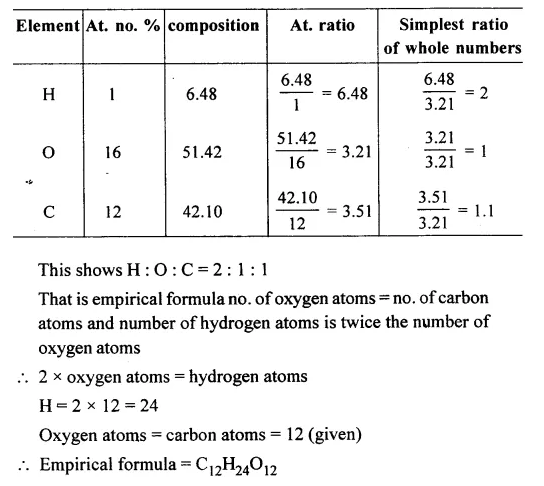
Empirical And Molecular Formula Chemistry Class 11 Some Basic Concepts Of Chemistry
It is also known as the simplest formula.
Empirical formula definition in math. Compressed Air - Pressure Drop in. But it is not possible always. Empirical formula A simple expression of the relative numbers of each type of atom in it or the simplest whole number ratio of atoms of each element present in a compound.
To calculate empirical. The empirical formula of a compound is the simplest whole number ratio of atoms of each element in the compound. Examples of empirical formula The molecular formula of.
21112019 This is the precise definition of empirical probability. An empirical formula for a compound is the formula of a substance written with the smallest integer subscript. It may be the same as the compounds molecular formula sometimes.
Empirical equations are based on observations and experience rather than theories - and as a result. Inductance in an Air Filled Cylindrical Coil. The empirical formula of any compound is the simplest whole-number ratio of each individual type of atom in that compound.
Medical Definition of empirical formula. The units on the right side of an equation do not always correspond to the units on the left side. Empirical probability is probability based on data collected through an experiment or observation.
21012018 The empirical formula is the simplest formula for a compound which is defined as the ratio of subscripts of the smallest possible whole number of the elements present in the formula. It is determined using data. Experimental or empirical probability is the probability of an event based on the results of an actual experiment conducted several times.
Empirical formula A chemical formula that indicates the relative proportions of the elements in a molecule rather than the actual number of atoms of the elements. It can be calculated from information about the mass of each. Evaporation from a Water Surface.
Examples - Empirical Equations. In theoretical probability we assume that the probability of occurrence of any event is equally likely and based on that we predict the probability of an event. A chemical formula showing the simplest ratio of elements in a compound rather than the total number of atoms in the molecule CH2O is the empirical formula for glucose compare molecular formula.
The empirical formula of a compound may be simpler than its molecular formula which is a multiple of the empirical formula. 10082020 Empirical Formula Definition The empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. 20082002 A mathematical symbol is a figure or a combination of figures that is used to represent a mathematical object an action on mathematical objects a relation between mathematical objects or for structuring the other symbols that occur in a formula.
25082019 The empirical formula is the simplest formula of the relative ratio of elements in a compound. 27012020 Writing Empirical Formula Practice Problems Chemistry Textbook Empirical Rule 68 95 99 7 Simple Definition Statistics Math Echte Vater. But the number of atoms of an element is always unknown.
The empirical formula of a substance is the simplest whole number ratio of the atoms of each element present.